Potential Drug against New Coronaviruses: MK-8591 (EFDA)
This Chinese New Year just passed will be an unforgettable Spring Festival for all Chinese people. People had no time to celebrate the coming of the Year of 2020 before a sudden attack of new crown pneumonia, which has completely broken the festive atmosphere that should have been for this. On December 8th, 2019, the first patient with unexplained pneumonia appeared in Wuhan, and subsequent cases of pneumonia occurred throughout the country and even around the world. Tens of thousands of people have been diagnosed with the infection so far. Starting on January 23rd, 2020, Wuhan has unprecedentedly suspended public transportation and barred citizens from leaving Wuhan. Subsequently, all parts of the country actively responded to the prevention and treatment of new coronary pneumonia. On January 30th, 2020, the WHO (World Health Organization) has announced that Wuhan pneumonia, the new type of coronaviruses, is an Public Health Emergency of International Concern (PHEIC).
Under the leadership of the Chinese government, the entire Chinese people are actively engaged in the fight against new coronaviruses pneumonia. In addition to strict isolation prevention and controlling work, as a drug worker, we are more concerned about which drugs are effective against the new coronaviruses. Because the coronaviruses is a new type of virus, there is no ready specific drug currently.
The traditional development of new drugs from scratch is difficult to play a role in the treatment of this round of pneumonia because it takes too long. Finding it from the approved or clinical antiviral drug is a more effective method, which can also greatly reduce the waiting time for patients.
In the past period of time, some reported therapeutic drugs still need more clinical practice to prove their effectiveness. Currently, at least seven small-molecule drugs targeting viral RNA polymerase or protease have been developed at different clinical research stages.
The earliest reported drug with a certain effect on new coronaviruses infection was reported by Wang Guangfa, the Director of Respiratory and Critical Illness, Peking University First Hospital, and member of the expert group of the National Health Commission. It is a drug called "Lopinavir and Ritonavir Tablets" (Kelizhi). It is a compound preparation, the two main ingredients, lopinavir and ritonavir, are protease inhibitors. They can be combined with the catalytic site of HIV protease and interfere with the assembly process of the virus. Therefore, it is used as an antiviral drug. This drug is clinically suitable for the combination with other antiretroviral drugs to treat HIV infection. Lu Hongzhou, a well-known expert in the field of infection and secretary of the Party Committee of the Shanghai Public Health Clinical Center, told the media recently that the new coronaviruses and HIV are RNA viruses that share some common features in treatment. It is indeed found in the clinic that the application of the anti-AIDS drug Kelizhi was very effective to treat new pneumonia patients. In the process of virus replication and assembly, the new coronaviruses may use some similar protein functions as HIV. "If HIV-specific protease inhibitors such as lopinavir and ritonavir can also bind to coronaviruses protease to inhibit their normal function, they can exert anti-coronaviruses effects," said Lu Hongzhou.
Recently, after the first confirmed case in the United States was treated with Remdesivir as a specific medicine, the disease showed rapid remission. Remdesivir was originally a drug used to treat Ebola virus. In its previous studies, it has been shown to have inhibitory effects on a variety of viruses, such as coronaviruses, hepatitis C virus, HIV virus, and Ebola virus. In its clinical study of Ebola virus, the results were not ideal, but its safety was acceptable. Based on current clinical results, Gilead Sciences said it offered experimental Ebola therapies for a small percentage of patients infected with coronaviruses. It is reported that Gilead's drug will undergo phase III clinical trials of coronaviruses in China. It is hoped that it will be applied to Chinese patients as soon as possible to provide strong support for fighting the new coronaviruses.
In addition to the two drugs reported above, the vast number of researchers in China are also fighting day and night to screen existing drugs in various ways, including the protease inhibitors of 12 anti-HIV drugs like Indinavir, Saquinavir, Lopinavir, Carfilzomib, Ritonavir. 2 anti-respiratory syncytial virus drugs, 1 anti-human macrophage drug, 1 anti-schizophrenia drugs, 1 immunosuppressant and 2 other drugs.
From the current results and the above research content, it can be found that the current research ideas are mainly focused on seeking the drug that can also inhibit new coronaviruses from clinically and marketed antiviral drugs. Based on this thinking, we think that Merck's MK-8591 (EFDA) should also be worth looking forward to its effect against the new coronaviruses disease.
MK-8591, also known as EFDA, was first discovered by Japanese scientists in 2001 in search of compounds that improved the taste of soy sauce. After confirming its structure, it was found to be close to some anti-HIV drugs. After further testing, it was found that MK-8591 has very high activity against HIV.
In subsequent developments, Merck acquired the molecule from Yamasa in Japan. The drug is a nucleoside reverse transcriptase inhibitor, whose potency and longevity are due to its role in two stages of the virus's life cycle: prevent HIV from making copies of the DNA of genes in the cells of the human body, and at the same time prevent the virus from transcribing more HIV DNA in the cell.
In laboratory studies, it has shown extremely high anti-HIV activity and safety. The drug is mainly used to prevent the spread of HIV in women and their children. In previous animal studies, researchers at the University of North Carolina found that MK-8591 was effective in preventing HIV transmission through the vagina and mouth. At present, the clinical research of MK-8591 for the treatment of HIV is in clinical stage 2B. The results of clinical trials show that MK-8591 is expected to be the first oral HIV drug that can be taken weekly, either for treatment or prevention.
If the ability of MK-8591 to prevent transmission can be equally effective in the treatment of new coronaviruses, then our work against new coronaviruses will be greatly reduced. Therefore,it is worthwhile to expect its effectiveness and safety against other viruses, including new coronaviruses.
Structure of MK-8591 (EFDA):
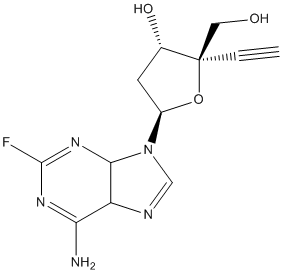 CAS# 865363-93-5
CAS# 865363-93-5
Under the leadership of the Chinese government, the entire Chinese people are actively engaged in the fight against new coronaviruses pneumonia. In addition to strict isolation prevention and controlling work, as a drug worker, we are more concerned about which drugs are effective against the new coronaviruses. Because the coronaviruses is a new type of virus, there is no ready specific drug currently.
The traditional development of new drugs from scratch is difficult to play a role in the treatment of this round of pneumonia because it takes too long. Finding it from the approved or clinical antiviral drug is a more effective method, which can also greatly reduce the waiting time for patients.
In the past period of time, some reported therapeutic drugs still need more clinical practice to prove their effectiveness. Currently, at least seven small-molecule drugs targeting viral RNA polymerase or protease have been developed at different clinical research stages.
The earliest reported drug with a certain effect on new coronaviruses infection was reported by Wang Guangfa, the Director of Respiratory and Critical Illness, Peking University First Hospital, and member of the expert group of the National Health Commission. It is a drug called "Lopinavir and Ritonavir Tablets" (Kelizhi). It is a compound preparation, the two main ingredients, lopinavir and ritonavir, are protease inhibitors. They can be combined with the catalytic site of HIV protease and interfere with the assembly process of the virus. Therefore, it is used as an antiviral drug. This drug is clinically suitable for the combination with other antiretroviral drugs to treat HIV infection. Lu Hongzhou, a well-known expert in the field of infection and secretary of the Party Committee of the Shanghai Public Health Clinical Center, told the media recently that the new coronaviruses and HIV are RNA viruses that share some common features in treatment. It is indeed found in the clinic that the application of the anti-AIDS drug Kelizhi was very effective to treat new pneumonia patients. In the process of virus replication and assembly, the new coronaviruses may use some similar protein functions as HIV. "If HIV-specific protease inhibitors such as lopinavir and ritonavir can also bind to coronaviruses protease to inhibit their normal function, they can exert anti-coronaviruses effects," said Lu Hongzhou.
Recently, after the first confirmed case in the United States was treated with Remdesivir as a specific medicine, the disease showed rapid remission. Remdesivir was originally a drug used to treat Ebola virus. In its previous studies, it has been shown to have inhibitory effects on a variety of viruses, such as coronaviruses, hepatitis C virus, HIV virus, and Ebola virus. In its clinical study of Ebola virus, the results were not ideal, but its safety was acceptable. Based on current clinical results, Gilead Sciences said it offered experimental Ebola therapies for a small percentage of patients infected with coronaviruses. It is reported that Gilead's drug will undergo phase III clinical trials of coronaviruses in China. It is hoped that it will be applied to Chinese patients as soon as possible to provide strong support for fighting the new coronaviruses.
In addition to the two drugs reported above, the vast number of researchers in China are also fighting day and night to screen existing drugs in various ways, including the protease inhibitors of 12 anti-HIV drugs like Indinavir, Saquinavir, Lopinavir, Carfilzomib, Ritonavir. 2 anti-respiratory syncytial virus drugs, 1 anti-human macrophage drug, 1 anti-schizophrenia drugs, 1 immunosuppressant and 2 other drugs.
From the current results and the above research content, it can be found that the current research ideas are mainly focused on seeking the drug that can also inhibit new coronaviruses from clinically and marketed antiviral drugs. Based on this thinking, we think that Merck's MK-8591 (EFDA) should also be worth looking forward to its effect against the new coronaviruses disease.
MK-8591, also known as EFDA, was first discovered by Japanese scientists in 2001 in search of compounds that improved the taste of soy sauce. After confirming its structure, it was found to be close to some anti-HIV drugs. After further testing, it was found that MK-8591 has very high activity against HIV.
In subsequent developments, Merck acquired the molecule from Yamasa in Japan. The drug is a nucleoside reverse transcriptase inhibitor, whose potency and longevity are due to its role in two stages of the virus's life cycle: prevent HIV from making copies of the DNA of genes in the cells of the human body, and at the same time prevent the virus from transcribing more HIV DNA in the cell.
In laboratory studies, it has shown extremely high anti-HIV activity and safety. The drug is mainly used to prevent the spread of HIV in women and their children. In previous animal studies, researchers at the University of North Carolina found that MK-8591 was effective in preventing HIV transmission through the vagina and mouth. At present, the clinical research of MK-8591 for the treatment of HIV is in clinical stage 2B. The results of clinical trials show that MK-8591 is expected to be the first oral HIV drug that can be taken weekly, either for treatment or prevention.
If the ability of MK-8591 to prevent transmission can be equally effective in the treatment of new coronaviruses, then our work against new coronaviruses will be greatly reduced. Therefore,it is worthwhile to expect its effectiveness and safety against other viruses, including new coronaviruses.
Structure of MK-8591 (EFDA):
 CAS# 865363-93-5
CAS# 865363-93-5







